 EZScreen™ Donor Specific Antibody detection
EZScreen™ Donor Specific Antibody detection
 Ricimer™ GMSbiotech Analysis software for EZMatch™ & EZScreen™ products
Ricimer™ GMSbiotech Analysis software for EZMatch™ & EZScreen™ products
 Transfusion Chip - Blood Antigens Genotyping
Transfusion Chip - Blood Antigens Genotyping
The "EZScreen™" Donor Specific Antibody screening and monitoring test product line will feature HLA Class I & II native antibody detection for pre-& post transplantation.
While traditional markers can aid in diagnosing the clinical status of solid organ transplant recipients, they are generally non-specific and most often only identifiable after graft damage has occurred. The early identification and subsequent removal of clinically harmful Donor Specific Antibodies (DSA) associated with Acute Mediated Rejection (AMR), both pre-& post-transplant may reduce allograft loss and improve patient outcomes.
"EZScreen™" is a sensitive and native state antibody screening and monitoring assay for the accurate detection of DSA for both pre-& post-transplant that is more cost-effective and easily fits into most labs current workflow. This platform is currently in development with Pure Transplant Solutions (PTS) LLC.
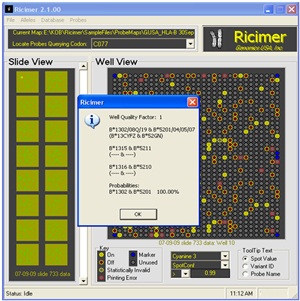
GMSbiotech Ricimer™ software is a companion to GMSbiotech's molecular typing and antibody screening products.
Analysis results can be reviewed in graphical form, allowing easy adjustment of cut-off values to clarify results. Data can be exported or formatted into custom reports. This software runs in both stand-alone (on a single computer) and network environments.
Features & Benefits
Blood group antigens are proteins found on the surface of red cells (erythrocytes) and platelets. They have traditionally been detected by serology in the agglutination test and are clinically very important in transfusion and transplantation. In both fields of medicine, the donor and recipient must have compatible blood group antigens, to avoid alloimmunization and, in the case of organ transplantation, graft rejection. Agglutination for blood typing has been automated and "gel cards" are now widely used in the developed world. However, once the genes encoding red blood cell antigens had been cloned and sequenced genotyping for red blood cell (RBC) antigens became feasible. There are several clinical applications where RBC genotyping has advantages over agglutination for the provision of safe blood. These include: (a) Patients who are recently transfused, (b) Patients who have a positive direct antiglobulin test because of antibodies bound to RBCs: such as in hemolytic anemia, (c) Presence of autoantibodies hinders serological RBC typing but not genotyping, or (d) Patient has a pre-existing clinically significant RBC antibody.
Genotyping is also extremely useful to confirm equivocal results or where there is evidence of weakly expressed antigens. In addition, there are worldwide shortages of 'traditional' grouping antisera that are leading to escalating test costs.
Clearly, there is an unmet need to combine the current understanding of blood group genetics with analytical technologies that would allow such genetics to be implemented, perhaps with field collection, as an inexpensive DNA-based test. We have adapted our microarray technology which was first developed for HLA, the most polymorphic protein and genetic system found in humans to develop the Transfusion Chip. This "Transfusion-Chip" or "T-Chip" for short will enable cost-effective genotyping for ABO, Rh and the other clinically important blood antigens to become the new standard for worldwide DNA-based Blood-Typing, by enabling both clinical and at-home sample collection. GMS technology allows the use of raw blood or cheekswab to carry out this testing.



| COUNTRY | PATENT NO. | GRANT DATE | EXPIRATION DATE | Available Fields of Use |
| United States of America | 7667026 | February 23, 2010 | February 27, 2027 | ALL |
| United States of America | 8,575,325 | November 5, 2013 | February 18, 2030 | ALL |
| Germany | 1991558 | November 18, 2015 | February 27, 2027 | ALL |
| France | 1991558 | November 18, 2015 | February 27, 2027 | |
| United Kingdom | 1991558 | November 18, 2015 | February 27, 2027 | |
| Australia | 2007313472 | May 1, 2014 | February 27, 2027 | |
| New Zealand | 597200 | October 1, 2013 | February 27, 2027 | ALL |
| New Zealand | 571513 | June 5, 2012 | February 27, 2027 | ALL |
| COUNTRY | PUB. NO. | PATENT NO. | GRANT DATE | EXPIRATION DATE | Available Fields of Use |
| United States of America | 7,354,710 | April 8, 2008 | July 11, 2022 | ALL | |
| United States of America | 10,272,409 | April 30, 2019 | July 11, 2022 | ALL | |
| United States of America | 20190209996 | ALL | |||
| Australia | 2009251843 | March 17, 2016 | April 3, 2029 | ALL | |
| Canada | 2,720,460 | October 29, 2019 | April 3, 2029 | ALL | |
| India | 300287 | August 27, 2018 | April 3, 2029 | ALL | |
| Japan | 6185958 | August 23, 2017 | May 7, 2035 | ALL | |
| Japan | 4312595 | August 12, 2009 | July 11, 2032 | ALL |
| COUNTRY | PUB. NO. | PATENT NO. | GRANT DATE | EXPIRATION DATE | Available Fields of Use |
| United States of America | 8771951 | July 8, 2014 | September 24, 2030 | ALL | |
| United States of America | 10,337,066 | July 2, 2019 | April 27, 2031 | ALL | |
| Australia | 2016266065 | September 19, 2019 | November 16, 2030 | ALL | |
| Belgium | 2,501,827 | December 20, 2017 | November 16, 2030 | ALL | |
| Canada | 2786118 | ALL | |||
| China | ZL201080060981 | January 20, 2016 | November 16, 2030 | ALL | |
| Germany | 2501827 | December 20, 2017 | November 16, 2030 | ALL | |
| European Patent Office | 3342880 | ALL | |||
| France | 2501827 | December 20, 2017 | November 16, 2030 | ALL | |
| United Kingdom | 2501827 | December 20, 2017 | November 16, 2030 | ALL | |
| Dem. People's Rep. of Korea | 10-1953176 | February 22, 2019 | November 16, 2030 | ALL |
| COUNTRY | PUB. NO. | PATENT NO. | GRANT DATE | EXPIRATION DATE | Available Fields of Use |
| United States of America | 9416419 | August 16, 2016 | September 24, 2030 | ALL | |
| United States of America | 20200080146 | ALL |
| COUNTRY | PUBLICATION NO. | Available Fields of Use |
| Patent Cooperation Treaty | WO 2020023484 | ALL |
|
COUNTRY |
PATENT NO. |
GRANT DATE |
EXPIRATION DATE |
Available Fields of Use |
| United States of America | 10,556,218 | February 11, 2020 | May 14, 2034 | EXCEPT HLA DIAGNOSTICS |
| Germany | 60 2014 036 584.1 | November 21, 2018 | May 14, 2034 | EXCEPT HLA DIAGNOSTICS |
| France | 2,997,037 | November 21, 2018 | May 14, 2034 | EXCEPT HLA DIAGNOSTICS |
| United Kingdom | 2,997,037 | November 21, 2018 | May 14, 2034 | EXCEPT HLA DIAGNOSTICS |
| United States of America | 9,751,069 | September 5, 2017 | June 6, 2034 | EXCEPT HLA DIAGNOSTICS |
Licensing opportunities are available for the above patents. Please contact Dr. Krishna Jayaraman.